| Pyridine hydrochloride Basic information |
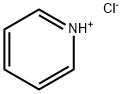
| Product Name: | Pyridine hydrochloride |
| Synonyms: | PYRIDINIUM MONOCHLORIDE;PYRIDINIUM CHLORIDE;PYRIDINE HYDROCHLORIDE;PYRIDINE HCL;Pyridine hydrochloride, 97+%;Pyridinehydrochloride,98%;PYRIDINE HYDROCHLORIDE PYRIDINE HYDROCHLORIDE;Pyridine hydrochloride, pure, 98% |
| CAS: | 628-13-7 |
| MF: | C5H6ClN |
| MW: | 115.56 |
| EINECS: | 211-027-4 |
| Product Categories: | Heterocycle-Pyridine series;Heterocyclic Compounds;Pyridinium Compounds;bc0001 |
| Mol File: | 628-13-7.mol |
 |
| Pyridine hydrochloride Chemical Properties |
| Melting point | 145-147 °C(lit.) |
| Boiling point | 222-224 °C(lit.) |
| density | 1.1182 (rough estimate) |
| refractive index | 1.5304 (estimate) |
| Fp | 222-224°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | ethanol: soluble50mg/mL, clear, colorless to light yellow |
| form | Crystals |
| color | White to tan |
| Water Solubility | 85 g/100 mL |
| Sensitive | Hygroscopic |
| BRN | 3615340 |
| Stability: | Hygroscopic |
| InChIKey | AOJFQRQNPXYVLM-UHFFFAOYSA-N |
| CAS DataBase Reference | 628-13-7(CAS DataBase Reference) |
| EPA Substance Registry System | Pyridine hydrochloride (628-13-7) |
| Safety Information |
| Hazard Codes | Xn,Xi |
| Risk Statements | 20/21/22-36/37/38-22 |
| Safety Statements | 26-36-37/39 |
| WGK Germany | 3 |
| RTECS | UT4375000 |
| F | 3-10 |
| TSCA | Yes |
| HS Code | 29333100 |
| Provider | Language |
|---|---|
| Pyridine hydrochloride | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Pyridine hydrochloride Usage And Synthesis |
| Chemical Properties | white to tan crystals |
| Uses | Pyridine Hydrochloride is the hydrochloride salt of pyridine, a basic six-membered heterocyclic ring. Pyridine is a base structure present in many biologically active compounds like the vitamins niaci n and pyridoxal. Pyridine is used in dehalogenation reactions and can be used as a base in condensation reactions. |
| Uses | Pyridine Hydrochloride is the hydrochloride salt of pyridine, a basic six-membered heterocyclic ring. Pyridine is a base structure present in many biologically active compounds like the vitamins niacin and pyridoxal. Pyridine is used in dehalogenation reactions and can be used as a base in condensation reactions. |
| Purification Methods | Crystallise the salt from CHCl3/EtOAc and wash it with Et2O. It is hygroscopic.[Beilstein 20 H 185, 20 I 57, 20 II 103, 20 III/IV 2230, 20/5 V 180.] |
| Pyridine hydrochloride Preparation Products And Raw materials |