| 2-Thiophenecarboxylic acid Basic information |
| Uses |
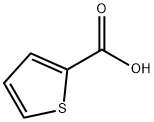
| Product Name: | 2-Thiophenecarboxylic acid |
| Synonyms: | 2-Thenoic Acid 2-Thiophenic Acid Thiophene-2-carboxylic acid;LABOTEST-BB LT02016392;AKOS B022791;AKOS BBS-00003739;ALPHA-THENOIC ACID;2-THENOIC ACID;2-THIOPHENIC ACID;2-THIOPHENECARBOXYLIC ACID |
| CAS: | 527-72-0 |
| MF: | C5H4O2S |
| MW: | 128.15 |
| EINECS: | 208-423-4 |
| Product Categories: | Building Blocks;C4 to C6;Heterocyclic Compounds;Miscellaneous;Chemical Synthesis;Heterocyclic Building Blocks;Thiophenes;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Thiophene&Benzothiophene;Organic acids;Building Blocks;Heterocyclic Building Blocks |
| Mol File: | 527-72-0.mol |
 |
| 2-Thiophenecarboxylic acid Chemical Properties |
| Melting point | 125-127 °C(lit.) |
| Boiling point | 260 °C(lit.) |
| density | 1.42 |
| refractive index | 1.5160 (estimate) |
| Fp | 259-261°C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Acetone (Slightly), Chloroform (Slightly) |
| form | solid |
| pka | 3.49(at 25℃) |
| color | White to Off-White |
| Water Solubility | 80 g/L (20 ºC) |
| Merck | 14,9354 |
| BRN | 110150 |
| InChIKey | QERYCTSHXKAMIS-UHFFFAOYSA-N |
| LogP | 1.570 |
| CAS DataBase Reference | 527-72-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Thiophenecarboxylic acid(527-72-0) |
| EPA Substance Registry System | 2-Thiophenecarboxylic acid (527-72-0) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36-37/39 |
| WGK Germany | 3 |
| RTECS | XM8330200 |
| Hazard Note | Irritant |
| TSCA | Yes |
| HS Code | 29349990 |
| Provider | Language |
|---|---|
| Thiophene-2-carboxylic acid | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| 2-Thiophenecarboxylic acid Usage And Synthesis |
| Uses | 2-Thenoic Acid, is a sulfur-containing heterocyclic compound, used in the synthesis of pharmaceutical and biologically active compounds. It is also shown to be utilized by presumptive Rhodococcus bacterium in soil, as a sole source of carbon. |
| Chemical Properties | white to light yellow crystal powder |
| Uses | 2-Thenoic Acid, is a sulfur-containing heterocyclic compound, used in the synthesis of pharmaceutical and biologically active compounds. It is also shown to be utilized by presumptive Rhodococcus bacterium in soil, as a sole source of carbon. |
| Definition | ChEBI: A thiophenecarboxylic acid in which the carboxy group is located at position 2. |
| Synthesis Reference(s) | The Journal of Organic Chemistry, 30, p. 3520, 1965 DOI: 10.1021/jo01021a055 |
| Purification Methods | Crystallise the acid from water and dry it in a vacuum. The amide has m 181o(from H2O) and pK2 5 10.54(50% aqueous dioxane). [Beilstein 18 H 289, 18 I 438, 18 II 269, 18 III/IV 4011, 18/6 V 158.] |
| 2-Thiophenecarboxylic acid Preparation Products And Raw materials |