| 2-Chlorothiophene Basic information |
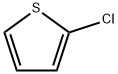
| Product Name: | 2-Chlorothiophene |
| Synonyms: | ChloroThiophene;2-Chlorothiophene,97%;2-Chlorothiphene;2 – chloride thiophene;2-Chlorothiophene, 98+%;2-Chlorothiophene, 98+% 25ML;2-CHLOROTHIOPHENE;2-THIENYL CHLORIDE |
| CAS: | 96-43-5 |
| MF: | C4H3ClS |
| MW: | 118.58 |
| EINECS: | 202-505-3 |
| Product Categories: | 12;Thiophene&Benzothiophene;Thiophenes;CHIRAL CHEMICALS;Heterocycle-oher series |
| Mol File: | 96-43-5.mol |
 |
| 2-Chlorothiophene Chemical Properties |
| Melting point | -71.9 °C |
| Boiling point | 127-129 °C (lit.) |
| density | 1.286 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.547(lit.) |
| Fp | 73 °F |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform |
| form | Liquid |
| color | Clear colorless to light brown |
| Specific Gravity | 1.286 |
| Water Solubility | INSOLUBLE |
| BRN | 104652 |
| Stability: | Volatile |
| InChIKey | GSFNQBFZFXUTBN-UHFFFAOYSA-N |
| CAS DataBase Reference | 96-43-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Thiophene, 2-chloro-(96-43-5) |
| EPA Substance Registry System | Thiophene, 2-chloro- (96-43-5) |
| Safety Information |
| Hazard Codes | Xi,F,T |
| Risk Statements | 10-36/37/38-23/24/25-37 |
| Safety Statements | 16-26-36-45-38-36/37/39-28B-7/9 |
| RIDADR | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| RTECS | XM8575000 |
| Hazard Note | Irritant |
| TSCA | T |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29349990 |
| Provider | Language |
|---|---|
| 2-Chlorothiophene | English |
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| 2-Chlorothiophene Usage And Synthesis |
| Chemical Properties | Colorless to light yellow liquid |
| Uses | 2-Chlorothiophene was used in the preparation of 5-phenylthiophene derivative. |
| Application | 2-Chlorothiophene is a heterocyclic compound that belongs to the group of organic compounds called thiophenes. It is an effective analgesic and anti-inflammatory drug that has been used in the treatment of inflammatory bowel disease. 2-Chlorothiophene is used as a reagent in chemical synthesis, especially the preparation of pharmaceutical preparations. This compound can be prepared by photoelectron or hydrochloric acid. The suzuki coupling reaction with pyrimidine compounds is another method for synthesizing this compound. |
| General Description | The photodissociation dynamics of 2-chlorothiophene was studied using resonance enhanced multiphoton ionization (REMPI) time-of-flight (TOF) technique. |
| Purification Methods | Purify it by fractional distillation at atmospheric pressure or by gas chromatography. [Conde et al. Synthesis 412 1976, Beilstein 17/1 V 303.] |
| 2-Chlorothiophene Preparation Products And Raw materials |