| (1S,2S,3R,5S)-(+)-2,3-Pinanediol Basic information |
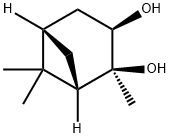
| Product Name: | (1S,2S,3R,5S)-(+)-2,3-Pinanediol |
| Synonyms: | [1S-(1α,2α,3α,5α)]-2,6,6-Trimethylbicyclo[3.1.1]heptane-2,3-diol;(1S, 2S, 3R, 5S)-(+)Pinanediol (Bortezomib Intermediate);(1S,2S,3R,5S)-(+)-2;cis-2,3-Pinandiol;(+)-2-Hydroxyisopinocampheol, (1S,2S,3R,5S)-2,3-Pinanediol, (1S,2S,3R,5S)-2,6,6-Trimethylbicyclo[3.1.1]heptane-2,3-diol, [1S-(1α,2α,3α,5α)]-2,6,6-Trimethylbicyclo[3.1.1]heptane-2,3-diol;(+)-2-Hydroxyisopinocampheol, (1S,2S,3R,5S)-2,6,6-Trimethylbicyclo[3.1.1]heptane-2,3-diol;(+)-pinanendiol;(1S,2S,3R,5S)-(+)-Pinanediol, (1S,2S,3R,5S)-(+)-2,3-Dihydroxy-2,6,6-trimethylbicyclo[3.1.1]heptane |
| CAS: | 18680-27-8 |
| MF: | C10H18O2 |
| MW: | 170.25 |
| EINECS: | 606-096-6 |
| Product Categories: | Chiral Compounds |
| Mol File: | 18680-27-8.mol |
 |
| (1S,2S,3R,5S)-(+)-2,3-Pinanediol Chemical Properties |
| Melting point | 57-59 °C(lit.) |
| alpha | 8.5 º (c=6.5, toluene) |
| Boiling point | 239.9°C (rough estimate) |
| density | 0.9409 (rough estimate) |
| refractive index | 1.4494 (estimate) |
| Fp | >230 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform, Methanol (Slightly), Toluene (Slightly) |
| pka | 14.68±0.60(Predicted) |
| form | Crystalline Powder, Crystals or Mass |
| color | White to light beige |
| optical activity | [α]20/D +8.5°, c = 6.5 in toluene |
| BRN | 1853591 |
| LogP | 1.582 (est) |
| CAS DataBase Reference | 18680-27-8(CAS DataBase Reference) |
| NIST Chemistry Reference | (1S,2s,3r,5s)-(+)-pinanediol(18680-27-8) |
| Safety Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| ACROS | English |
| (1S,2S,3R,5S)-(+)-2,3-Pinanediol Usage And Synthesis |
| Chemical Properties | white to light beige crystalline powder, crystals |
| Uses | (1S,2S,3R,5S)-(+)-2,3-Pinanediol is a bicyclic monoterpene diols that has been shown to induce differentiation of S91 melanoma and PC12 pheochromocytoma cells and thus provide possible therapeutic and sunless tanning use. It is also used in the transformation of isopinocampheol and caryophyllene oxide. |
| Uses | (1S,2S,3R,5S)-(+)-2,3-Pinanediol is a bicyclic monoterpene diols that has been shown to induce differentiation of S91 melanoma and PC12 pheochromocytoma cells and thus provide possible therapeutic and sunless tanning use. It is also used in the transformation of isopinocampheol and caryophyllene oxide. |
| Uses | (1S,2S,3R,5S)-(+)-Pinanediol can be used as a chiral reagent in the synthesis of homochiral α-hydroxyketones and in the resolution of prolineboronate esters. |
| (1S,2S,3R,5S)-(+)-2,3-Pinanediol Preparation Products And Raw materials |
| Raw materials | Sodium metabisulfite–>Sodium thiosulfate pentahydrate–>Osmium tetroxide–>4-METHYLMORPHOLINE-4-OXIDE SOLUTION–>(1R)-(+)-ALPHA-PINENE–>TRIETHANOLAMINE BORATE–>Triethanolamine |
| Preparation Products | (+)-PINANEBORANE |