| Chemical Properties | White crystals or crystalline powder. Decomposed by moisture at temperatures around 40C. Soluble in water, alcohol, and ethylene glycol; solvents such as ether and acetone extract hydrogen peroxide and may form explosive solutions. Active oxygen (min) 16% |
| Uses | For extemporaneous preparation of H2O2. |
| Uses | Urea hydrogen peroxide is an antiseptic, disinfectant and bleaching agent used in pharmaceuticals and cosmetics respectively. It is used in the whitening of teeth and relieves minor inflammation of gums, oral mucosal surfaces and lips. It finds application in the preparation of plastics, blue print development and starch modification. In addition to this, it is used as a source of hydrogen peroxide easily handled in the laboratory. |
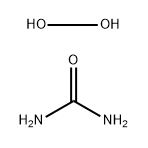
| Definition | ChEBI: A mixture obtained by combining equimolar amounts of hydrogen peroxide and urea. |
| Brand name | Murine Ear Drops (Ross). |
| General Description | A solid or paste-like semisolid. Used to make plastics. |
| Air & Water Reactions | Decomposed by moisture at about 40°C to yield a solution of hydrogen peroxide (nonhazardous reaction). Water soluble. |
| Reactivity Profile | Urea hydrogen peroxide is an oxidizing agent. Liable to spontaneous combustion when heated or in contact with organic materials. The contents of a screw-capped brown glass bottle spontaneously erupted after four years storage at ambient temperature. [MCA Case History No. 719]. Combustion may release Irritating ammonia gas. |
| Health Hazard | Inhalation of dust causes irritation of nose from hydrogen peroxide formed when heated. Contact with eyes causes severe damage. Contact with moist skin causes temporary itching or burning sensation. Ingestion causes irritation of mouth and stomach. |
| Flammability and Explosibility | Notclassified |
| Clinical Use | Carbamide peroxide (Gly-Oxide) is a stable complex of ureaand hydrogen peroxide. It has the molecular formulaH2NCONH2 H2O2. The commercial preparation is a solutionof 12.6% carbamide peroxide in anhydrous glycerin.When mixed with water, hydrogen peroxide is liberated.Carbamide peroxide is used as both an antiseptic and disinfectant.The preparation is especially effective in the treatmentof oral ulcerations or in dental care. The oxygen bubblesthat are liberated remove debris. |
| Purification Methods | It is a safe alternative to H2O2 in various oxidation reactions. It is commercially available in tablets (“rapidly soluble”, equivalent to ~30% H2O2) or as a white powder (with 15-17% active oxygen). It is usually used without purification after assaying for active oxygen. This is done by titration with potassium permanganate or by iodometry, i.e. titration of liberated iodine when glacial acetic acid containing Fe3+ and NaI are added. It can be recrystallised from 30% H2O2 in a molar ratio of ~2:3 by heating in a pyrex dish for a few minutes at ~60o, cooling and allowed to crystallise slowly by evaporation in a crystallising dish. It forms elongated white needles, but if the solution is seeded just before crystallisation and shaken gently for as few seconds, then small plates are formed. Perferably collect the crystals by centrifugation at low temperature and dry them at 0o in vacuo. When dry, it is stable at room temperature and it has been reported that the available oxygen content had not decreased noticeably after 12 months. However, it is best to store it dry at low temperature. It is soluble in organic solvents e.g. EtOH, Et2O, CHCl3, CH2Cl2 and Me2CO with slow decomposition, and its solubility in H2O is 40% where it also decomposes slowly. It decomposes slowly at 40-60o/20mm and at 55-70o/760mm in air, but decomposition appears to accelerate above 80o. It is very useful (and in many cases superior to p-chloroperbenzoic acid) in the oxidation of alkenes, (epoxides), aromatic hydrocarbons (to phenols), ketones (Baeyer-Villiger), sulfides (to sulfones) and N-heterocycles (to N-oxides) when using 5 to 10 molar ratios of oxidant in the presence of acetic or trifluoroacetic anhydrides. Care should be used with this reagent as it is potentially explosive. [Lu et al. J Am Chem Soc 63 1508 1941, Cooper et al. Synlett 533 1990, Beilstein 3 H 54, 3 I 25, 3 II 45, 3 III 105, 3 IV 102.] |