| Chemical Properties | white crystalline powder |
| Uses | Convenient reagent for the reduction of ketones to secondary alcohols.
Thiourea dioxide is an effective bleach when used alone or when used after hydrogen peroxide in a full bleaching process (Duffield, 1986; Cegarra et al, 1988). Bleaching with thiourea dioxide is not common practice but it is effective when used alone, and the process compares favorably with hydrogen peroxide bleaching. A formulation can include a commercial thiourea dioxide product, wetting agent and EDTA sequestering agent. Reductive bleaching is carried out at pH 7.0 at 70°C for 60 min (Duffield, 1986).
 |
| Uses | Convenient reagent for the reduction of ketones to secondary alcohols. |
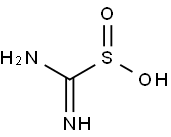
| General Description | A white or light-yellow odorless crystalline powder. Mp:126°C. Soluble in water (27 g / L at room temperature). Decomposes exothermically at temperatures above 126°C with the emission of noxious gases (sulfur oxides, ammonia, carbon monoxide, nitrogen oxides and hydrogen sulfide) and carbon dioxide. Extended exposure to temperatures above 50°C and moisture may cause visible decomposition. Irritating to skin and mucous membranes. Corrosive to eye tissue. Used in leather processing, the paper industry, photographic industry, and in textile processing as a bleaching agent. |
| Air & Water Reactions | Soluble in water |
| Reactivity Profile | Thiourea dioxide is a reducing agent and a derivative of sulfinic acid (a weak inorganic acid). Decolorizes and bleaches materials by chemical reduction. Stable under normal temperatures and pressures. May decompose on exposure to moist air or water. Incompatible with strong oxidizing agents, strong bases. Aqueous solutions are acidic and corrosive. |
| Health Hazard | Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution. |
| Fire Hazard | Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated. |
| Flammability and Explosibility | Nonflammable |
| Purification Methods | Dissolve it in five parts of aqueous 1:1% NaHSO3 at 60-63o (charcoal), then allow it to crystallise slowly, with agitation, at 10o. Filter and dry it immediately at 60o [Koniecki & Linch Anal Chem 30 1134 1958]. [Beilstein 3 I 36, 3 IV 145.] |