| HAFNIUM Basic information |
| History, Occurrence and Uses Chemical Properties Physical Properties Uses Production Reactions |
| Product Name: | HAFNIUM |
| Synonyms: | celtium;hafniummetal,dry(dot);hafniummetal,wet(dot);hafniumpowder;HAFNIUM POWDER, -60+325 MESH, 99.6% (METALS BASIS EXCLUDING;HAFNIUM WIRE, 0.25MM (0.01IN) DIA, 99.97% (METALS BASIS EXCL;HAFNIUM CRYSTAL BAR, 99.7% (METALS BASIS EXCLUDING ZR), ZR N;HAFNIUM FOIL, 0.75MM (0.03IN) THICK, 99.5% (METALS BASIS EXC |
| CAS: | 7440-58-6 |
| MF: | Hf |
| MW: | 178.49 |
| EINECS: | 231-166-4 |
| Product Categories: | Inorganics;metal or element;Hafnium;Metal and Ceramic Science;Metals |
| Mol File: | 7440-58-6.mol |
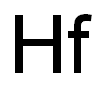 |
| HAFNIUM Chemical Properties |
| Melting point | 2227 °C (lit.) |
| Boiling point | 4602 °C (lit.) |
| density | 13.3 g/cm3 (lit.) |
| storage temp. | Store at +15°C to +25°C. |
| form | wire |
| Specific Gravity | 13.31 |
| color | Silver-gray |
| resistivity | 29.6 μΩ-cm, 0°C |
| Water Solubility | soluble HF; slowly reacts with conc H2SO4, aqua regia [KIR80] |
| Merck | 13,4603 |
| Exposure limits | ACGIH: Ceiling 2 ppm OSHA: Ceiling 5 ppm(7 mg/m3) NIOSH: IDLH 50 ppm; Ceiling 5 ppm(7 mg/m3) |
| Stability: | Stable. Incompatible with oxygen, sulfur, strong oxidizing agents, halogens, phosphorus, strong acids. Highly flammable. |
| InChIKey | VBJZVLUMGGDVMO-UHFFFAOYSA-N |
| CAS DataBase Reference | 7440-58-6(CAS DataBase Reference) |
| EPA Substance Registry System | Hafnium (7440-58-6) |
| Safety Information |
| Hazard Codes | F,Xn,T |
| Risk Statements | 11-20/21/22-34-23/24/25 |
| Safety Statements | 9-16-26-27-33-36-36/37/39-45-28 |
| RIDADR | UN 3178 4.1/PG 3 |
| WGK Germany | – |
| RTECS | MG4600000 |
| TSCA | Yes |
| HS Code | 3822 00 00 |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 7440-58-6(Hazardous Substances Data) |
| IDLA | 50 mg Hf/m3 |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| HAFNIUM Usage And Synthesis |
| History, Occurrence and Uses | Hafnium was discovered in 1922 by Coster and deHevesy. They named it for Hafnia, the Latin word for Copenhagen. It is found in all zirconium ores, such as zircon, (ZrSiO4) and baddeleyite (ZrO2). It occurs in the earth’s crust at about 3 mg/kg. Its average concentration in sea water is 7 ng/L. Hafnium is used in control rods for nuclear reactors. It has high resistance to radiation and also very high corrosion resistance. Another major application is in alloys with other refractory metals, such as, tungsten, niobium and tantalum. |
| Chemical Properties | Hafnium is a lustrous silvery, ductile metal typically found in the 4+ oxidation state. It is poorly soluble in water as a pure metal. It is relatively unreactive to alkalis and strong acids, except hydrofluoric acid and is frequently alloyed with iron, niobium, tantalum, and titanium. Pure hafnium power is pyrophoric and may spontaneously ignite in air, especially under conditions of high moisture. Hafnium powder reacts violently with strong oxidizers and strong acids (Pohanish, 2011). When heated to around 200 °C, hafnium metal may react with several period 2 inorganic elements as well as silicone and sulfur. Hafnium oxide (HfO2) is poorly soluble in water and is a refractory compound, which is highly resistant to corrosion. With halogens, it may react to form tetrahalides. |
| Physical Properties | Occurs as a close-packed hexagonal alpha-form and a body-centered cubic beta modification; electrical resistivity 35.5 microhm-cm at 20°C; magnetic susceptibility 0.42×10–6 emu/g at 25°C; thermal neutron absorption cross section 105 barns/atom; work function 3.5 eV; modulus of elasticity 20×106 psi; tensile strength 58,000 psi at 25°C; insoluble in water, dilute mineral acids and nitric acid at all concentrations; soluble in hydrofluoric acid, concentrated sulfuric acid and aqua regia. |
| Uses | Hafnium use is limited due to low abundance. The primary use of hafnium is in the nuclear industry, where it is used in fuel rods to regulate fission given its high neutron absorption cross section. Similar to zirconium, hafnium is alloyed with niobium and carbide to produce high temperature refractory materials for furnaces and jet components as well as for plasma cutters. In addition, hafnium oxide is increasingly being used to augment or replace silicone oxidebased microprocessor chips in certain applications as well as in cathodes and capacitors (Field et al., 2011). |
| Production | Hafnium is obtained commercially from mineral zircon, which is zirconium orthosilicate [14940-68-2]. Zircon usually contains hafnium oxide, HfO2, in an amount that ranges between 1 to 2%. Zircon sand is separated from heavy mineral fractions from alluvial deposits by various electrostatic and magnetic separation processes. The sand is then ground and heated with caustic soda at 600°C or with soda ash at 1,000°C, or fused with lime at elevated temperatures to separate silicates. Alternatively, zircon may be decomposed by heating with chlorine in the presence of coke at 1,100°C. In the caustic fusion process, pulverized fusion cake is washed with water to remove water-soluble sodium silicate and unreacted caustic soda, leaving behind insoluble hydrous zirconium oxide. Hydrous zirconium oxide is soluble in most acids. It is dissolved in hydrochloric acid and filtered to remove unreacted ore and silica. When the chlorination process is applied, the products are zirconium tetrachloride, hafnium tetrachloride, and silicon tetrachloride. Silicon tetrachloride is more volatile than the other two chlorides and, therefore, zirconium tetrachloride and hafnium tetrachloride can be removed from silicon tetrachloride by condensing under controlled heating. The condensed tetrachlorides are dissolved in water and filtered to remove insoluble matter. |
| Reactions | The chemical properties of hafnium are very much similar to those of zirconium. In aqueous solutions, the metal exists in tetravalent state. The electrode potential for the reaction Hf→ Hf 4+ + 4e¯ is –1.70V. The metal in bulk form does not react with most reagents at ordinary temperatures. However, the powdered metal or hafnium sponge may readily burn in air after ignited with a spark. When heated at 360°C under water pressure, the metal is oxidized to hafnium oxide, forming a thin, protective, surface oxide layer. A similar surface hafnium oxide layer forms in nitric acid, which protects the metal from acid attack. Reaction with hydrofluoric acid at ordinary temperatures yields hafnium tetrafluoride, HfF4. Reaction with hydrogen occurs around 700°C. Hafnium absorbs rapidly, forming a hydride which probably has a composition HfH1.86. Hafnium metal reacts very slowly in concentrated sulfuric acid at ordinary temperatures. At acid concentration above 70% and under boiling conditions, sulfuric acid readily attacks the metal. |
| Chemical Properties | Hafnium is a refractory metal which occurs in nature in zirconium minerals. |
| Physical properties | Hafnium is a ductile metal that looks and feels much like stainless steel, but it is significantlyheavier than steel. When freshly cut, metallic hafnium has a bright silvery shine. Whenthe fresh surface is exposed to air, it rapidly forms a protective oxidized coating on its surface.Therefore, once oxidized, hafnium resists corrosion, as do most transition metals, whenexposed to the air. Chemically and physically, hafnium is very similar to zirconium, whichis located just above it in group 4 on the periodic table. In fact, they are so similar that it isalmost impossible to secure a pure sample of either one without a small percentage of theother. Each will contain a small amount of the other metal after final refining. Hafnium’s melting point is 2,227°C, its boiling point varies from about 2,500°C to5,000°C depending on its purity, and its density is 13.29 g/cm3. The compound hafniumnitride (HfN) has the highest melting point (over 3,300°C) of any two-element compound. |
| Isotopes | There are 44 known isotopes for hafnium. Five are stable and one of the unstableisotopes has such a long half-life (Hf-174 with a 2.0×10+15 years) that it is includedas contributing 0.16% to the amount of hafnium found in the Earth’s crust. The percentagecontributions of the 5 stable isotopes to the element’s natural existence on Earth areas follows: Hf-176 = 5.26%, Hf-177 = 18.60%, Hf-178 = 27.28%, Hf-179 = 13.62%,and Hf-180 = 35.08%. |
| Origin of Name | Named after Hafnia, the Latin name for the city of Copenhagen, Denmark. |
| Occurrence | Hafnium is the 47th most abundant element on Earth. Thus, it is more abundant thaneither gold or silver. Because hafnium and zirconium are always found together in nature, bothmetals are refined and produced by the Kroll process. Pure samples of either hafnium or zirconiumare almost impossible to separate by the Kroll or other refining processes. Baddeleyite(ZrO2), a zirconium ore, and zircon (ZrSiO4) are treated with chlorine along with a carboncatalyst that produces a mixture of zirconium and hafnium tetrachlorides. These are reducedby using sodium or magnesium, resulting in the production of both metals. The molten metalsare separated by the process known as fractionation, which depends on their different meltingpoints and densities. As the mixture of the two metals cools during the fractionation process,the denser solidified hafnium sinks to the bottom of the vessel while the less dense zirconium(with a higher melting point than hafnium) floats on top. |
| History | Hafnium was thought to be present in various minerals and concentrations many years prior to its discovery, in 1923, credited to D. Coster and G. von Hevesey. On the basis of the Bohr theory, the new element was expected to be associated with zirconium. It was finally identified in zircon from Norway, by means of X-ray spectroscopic analysis. Hafnium was named in honor of the city in which the discovery was made. Most zirconium minerals contain 1 to 5% hafnium. It was originally separated from zirconium by repeated recrystallization of the double ammonium or potassium fluorides by von Hevesey and Jantzen. Metallic hafnium was first prepared by van Arkel and deBoer by passing the vapor of the tetraiodide over a heated tungsten filament. Almost all hafnium metal now produced is made by reducing the tetrachloride with magnesium or with sodium (Kroll Process). Hafnium is a ductile metal with a brilliant silver luster. Its properties are considerably influenced by the impurities of zirconium present. Of all the elements, zirconium and hafnium are two of the most difficult to separate. Their chemistry is almost identical; however, the density of zirconium is about half that of hafnium. Very pure hafnium has been produced, with zirconium being the major impurity. Natural hafnium contains six isotopes, one of which is slightly radioactive. Hafnium has a total of 41 recognized isotopes and isomers. Because hafnium has a good absorption cross section for thermal neutrons (almost 600 times that of zirconium), has excellent mechanical properties, and is extremely corrosion resistant, it is used for reactor control rods. Such rods are used in nuclear submarines. Hafnium has been successfully alloyed with iron, titanium, niobium, tantalum, and other metals. Hafnium carbide is the most refractory binary composition known, and the nitride is the most refractory of all known metal nitrides (m.p. 3310°C). Hafnium is used in gas-filled and incandescent lamps, and is an efficient “getter” for scavenging oxygen and nitrogen. Finely divided hafnium is pyrophoric and can ignite spontaneously in air. Care should be taken when machining the metal or when handling hot sponge hafnium. At 700°C hafnium rapidly absorbs hydrogen to form the composition HfH1.86. Hafnium is resistant to concentrated alkalis, but at elevated temperatures reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Halogens react directly to form tetrahalides. The price of the metal is about $2/g. The yearly demand for hafnium in the U.S. is now in excess of 50,000 kg. |
| Characteristics | As the first element in the third series of the transition elements, hafnium’s atomic number(72Hf ) follows the lanthanide series of rare-earths. The lanthanide series is separated out ofthe normal position of sequenced atomic numbers and is placed below the third series on theperiodic table (57La to 71Li). This rearrangement of the table allowed the positioning of elementsof the third series within groups more related to similar chemical and physical characteristics—for example, the triads of Ti, Zr, and Hf; V, Nb, and Ta; and Cu, Ag, and Au. |
| Uses | Hafnium has a great affinity for absorbing slow neutrons. This attribute, along with itsstrength and resistance to corrosion, makes it superior to cadmium, which is also used formaking control rods for nuclear reactors. This use is of particular importance for the type ofnuclear reactors used aboard submarines. By moving the control rods in and out of a nuclearreactor, the fission chain reaction can be controlled as the neutrons are absorbed in the metalof the rods. The drawback to hafnium control rods is their expense: it costs approximately onemillion dollars for several dozen rods for use in a single nuclear reactor. In vacuum tubes and other applications that must have gases removed, hafnium is used asa “getter” to absorb any trace oxygen or nitrogen in the tube, thus extending the life of thevacuum tube. Hafnium’s qualities also make it ideal for filaments in light bulbs and, whenmixed with rare-earth metals, as a “sparking” misch metal. Hafnium is also used to a lesserextent as an alloying agent for several other metals, including iron, titanium, and niobium. |
| Uses | Obtained in mining and purification of the metal; used in control rods in nuclear reactors and in manufacture of light bulb filaments; found in all zirconiumcontaining minerals |
| Definition | hafnium: Symbol Hf. A silvery lustrousmetallic transition element;a.n. 72; r.a.m. 178.49; r.d. 13.3; m.p.2227±20°C; b.p. 4602°C. The elementis found with zirconium and is extractedby formation of the chlorideand reduction by the Kroll process. Itis used in tungsten alloys in filamentsand electrodes and as a neutron absorber.The metal forms a passiveoxide layer in air. Most of its compoundsare hafnium(IV) complexes;less stable hafnium(III) complexesalso exist. The element was first reportedby Urbain in 1911, and its existencewas finally established by Dirk Coster (1889–1950) and Georgede Hevesey (1885–1966) in 1923. |
| General Description | HAFNIUM, is a grayish metallic colored powder. Dust from dry powder may be ignited by static electricity. The dry powder reacts with moisture to produce hydrogen, a flammable gas. The heat from this reaction may be sufficient to ignite the hydrogen. HAFNIUM does not appreciably react with large quantities of water. |
| Air & Water Reactions | Highly flammable. The dry powder reacts with moisture to produce hydrogen, a flammable gas. The heat from this reaction may be sufficient to ignite the hydrogen. HAFNIUM does not appreciably react with large quantities of water. |
| Reactivity Profile | Metals, such as HAFNIUM METAL(reactivity similar to zirconium), are reducing agents and tend to react with oxidizing agents. Their reactivity is strongly influenced by their state of subdivision: in bulk they often resist chemical combination; in powdered form they may react very rapidly. Thus, as a bulk metal HAFNIUM is somewhat unreactive, but finely divided material may be pyrophoric. The metal reacts exothermically with compounds having active hydrogen atoms (such as acids and water) to form flammable hydrogen gas and caustic products. The reactions are less vigorous than the similar reactions of alkali metals, but the released heat can still ignite the released hydrogen. Materials in this group may react with azo/diazo compounds to form explosive products. These metals and the products of their corrosion by air and water can catalyze polymerization reactions in several classes of organic compounds; these polymerizations sometimes proceed rapidly or even explosively. Some metals in this group form explosive products with halogenated hydrocarbons. |
| Hazard | Although the metal hafnium is not harmful, its powder and dust are both toxic if inhaledand explosive even when wet. |
| Health Hazard | Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution. |
| Fire Hazard | Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated. |
| Flammability and Explosibility | Highlyflammable |
| Industrial uses | Pure hafnium is a lustrous, silvery metal that is not so ductile nor so easily worked as zirconium; nevertheless, hafnium can be hot- and cold-rolled on the same equipment and with similar techniques as those used for zirconium. All zirconium chemicals and alloys may contain some hafnium, and hafnium metal usually contains about 2% zirconium.The metal has a closepacked hexagonal structure. The electric conductivity is about 6% that of copper. It has excellent resistance to a wide range of corrosive environments. Because of the startling similarity in their chemical properties, zirconium and hafnium always occur together in nature. In their respective ability to absorb neutrons, however, they differ greatly, and this difference has led to their use in surprisingly different ways in nuclear reactors. Zirconium, with a low neutron-absorption cross section (0.18 barn), is highly desirable as a structural material in water-cooled nuclear reactor cores. Hafnium, on the other hand, because of its high neutron-absorption cross section (105 barns), can be used as a neutron-absorbing control material in the same nuclear reactor cores. Thus, the two elements, which occur together so intimately in nature that they are very difficult to separate, are used as individual and important but contrasting components in the cores of nuclear reactors. |
| Potential Exposure | Hafnium metal has been used as a control rod material in nuclear reactors. Thus, those engaged in fabrication and machining of such rods may be exposed. |
| Shipping | UN1326 Hafnium powder, wetted with not <,25% water (a visible excess of water must be present) (1) mechanically produced, particle size<53 μm; (2) chemically produced, particle size<840 μm, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN2545 Hafnium pow der, dry, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1346 Hafnium powder, wetted with not less than 25% water (a visible excess of water must be present) (1) mechanically produced, particle size less than 53 μm; (2) chemically produced, particle size less than 840 μm, Hazard Class: 4.1; Labels: 4.1-Flammable solid. |
| Incompatibilities | Fine powder or dust may form explosive mixture in air. The powder is highly flammable and a strong reducing agent. The powder or dust reacts with moisture forming flammable hydrogen gas; may spontaneously ignite on contact with moist air; and at higher temperatures, with nitrogen, phosphorous, oxygen, halogens, and sulfur; contact with hot nitric acid; heat, shock, friction, strong oxidizers; or ignition sources may cause explosions. |
| Waste Disposal | Recovery. Consider recycling, otherwise, this chemical must be disposed of in compliance with existing federal and local regulations. |
| HAFNIUM Preparation Products And Raw materials |