| Chemical Properties | Caravone occurs in different forms. l-Carvone exhibits odor of spearmint, while d-carvone exhibits odor reminiscent of caraway. |
| Chemical Properties | Carvone is a pale yellow to white clear liquid. |
| Chemical Properties | colourless or pale yellow liquid |
| Occurrence | The optically active and inactive forms have been reported among the constituents of about 70 essential oils. The dextro form is present in carvi, Antheum graveolens, Antheum sowa, Lippia carviodora, Mentha arvensis, etc. The levo form is present in Metha vifidis var. crispa, Mentha longifolia from South Africa, Eucalyptus globules and several mint species. The racemic form is present in ginger grass, Litsea gutalemaleusis, lavender and Artemisia ferganensis. Reported found in citrus oil and juice (lemon, lime, orange), celery seed, anise, clove, coriander seed, calamus, caraway herb and dill seed. |
| Uses | (S)-(+)-Carvone can be used as a starting material to synthesize:
(-)-Samaderine Y, a pentacyclic quassinoid.(-)-Ambrox, a terpenoid responsible for the odor of ambergris.3β-Acetoxydrimenin (a sesquiterpene) via conjugated addition of potassium cyanide followed by base catalyzed Robinson annulation reaction.Thapsigargin family members such as trilobolide, nortrilobolide, and thapsivillosin F. |
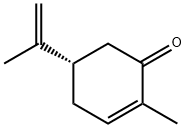
| Definition | ChEBI: A carvone having (S) configuration. |
| Aroma threshold values | Detection: d-Carvone: 6.7 to 820 ppb; l-carvone: 2.7 to 600 ppb |
| General Description | Pale yellow or colorless liquid. |
| Air & Water Reactions | May be sensitive to prolonged exposure to light and air. Insoluble in water. |
| Reactivity Profile | D(+)-Carvone may be sensitive to prolonged exposure to light and air. Incompatible with strong oxidizing agents and strong reducing agents |
| Fire Hazard | D(+)-Carvone is combustible. |
| Flammability and Explosibility | Notclassified |
| Safety Profile | Poison by ingestion and skin contact. A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Synthesis | Carvone occurs in the dextro, levo and racemic form; l-carvone can be isolated from the essential oil of spearmint or is commercially synthesized from d-limonene; d-carvone is usually prepared by fractional distillation of oil of caraway, also from dillseed and dillweed oils, but this type differs in odor and flavors. |
| Potential Exposure | Carvone is found in various natural oils, including caraway and dillseed; mandarin peel and spearmint oils. A food additive, it is used in flavoring liqueurs; in perfumes and soaps. |
| Shipping | UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required |
| Incompatibilities | Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. |