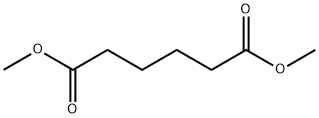
| Description | Dimethyl adipate (DMA) is a colourless and flammable liquid. It is soluble in alcohol and ether but sparingly soluble in water. DMA is incompatible with strong oxidising agents, and on decomposition, it emits carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide. DMA reacts with acids, alkalis, and strong oxidants. DMA is synthesised by the esterification of adipic acid. DMA is part of a dibasic ester (DBE) blend used as a major ingredient in several paint strippers, and the DBE blends used in paint stripping formulations contain a major portion (about 90%) of DMA. DMA is used as a chemical intermediate (polymers, agrochemicals), cellulose resins, a speciality solvent (inks, coatings, adhesives), and an emollient and can also be utilised as a paint remover and plasticiser. |
| Chemical Properties | Dimethyl adipate is a colorless liquid. It is a fatty acid methyl ester. |
| Uses | Dimethyl adipate is used in cosmetics (in emollients and skin conditioning). It acts as a cosmetic plasticizer. It is also used in agrochemicals and dye as well as a precursor for the preparation of active pharmaceutical ingredients. Further, it serves as a polymer intermediate. In addition to this, it is employed as a solvent for paint stripping and resins. |
| Uses | Dimethyl adipate is used in cosmetics (in emollients and skin conditioning).It is used as a plasticizer for cellulose-type resins and a finish remover.It is also used in agrochemicals and dye as well as a precursor for the preparation of active pharmaceutical ingredients. Further, it serves as a polymer intermediate. In addition to this, it is employed as a solvent for paint stripping and resins. |
| Production Methods | Dimethyl adipate is manufactured via esterification of adipic acid and methanol in the presence of an acid catalyst. |
| Definition | ChEBI: Dimethyl adipate is a fatty acid methyl ester. |
| General Description | Colorless liquid. |
| Air & Water Reactions | Flammable. Hydrolyzed by strong mineral acids and strong alkalis. |
| Reactivity Profile | Dimethyl adipate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. |
| Health Hazard | Exposures to dimethyl adipate cause toxicity and adverse health effects in laboratory animals and humans. Workplace exposures to dimethyl adipate by inhalation, ingestion, or skin absorption cause harmful and irritation effects to users. |
| Health Hazard | May be harmful by inhalation, ingestion, or skin absorption. May cause irritation. |
| Flammability and Explosibility | Nonflammable |
| Safety Profile | Moderately toxic by intraperitoneal route. Experimental teratogenic and reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Synthesis | Dimethyl adipate was synthesized by immobilized Candida antarctica lipase B-catalyzed esterification of adipic acid and methanol.
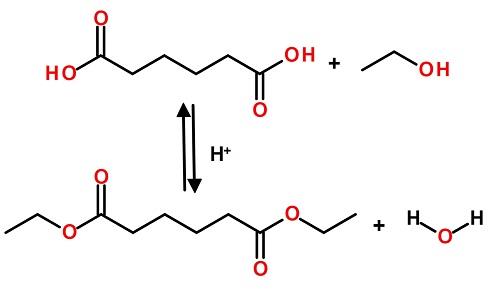
According to the general procedure described above, Dimethyl adipate has been synthesized from adipic acid (730mg, 5 mmol) and methanol (10 ml). Yield: 9%.
1H NMR (400.1 MHz, CDCl3): δ = 3.56 (6H, s, H1-H10), 2.23 (4H, m, H4-H7), 1.56 (4H, m, H5-H6).
13C NMR (100.5 MHz, CDCl3): δ = 173.4 (C3-C8), 51.2 (C1-C10), 33.4 (C4-C7), 24.1 (C5-C6)
https://pubmed.ncbi.nlm.nih.gov/20632329/ |
| Carcinogenicity | In a chronic inhalation toxicity study of dimethyl adipate, groups of male and female rats were exposed to 400 mg/m3 of dimethyl adipate over a 90-day period. Focal respiratory metaplasia of the olfactory epithelium was found. These nonneoplastic lesions were minimal to mild in severity . |
| Precautions | During handling of dimethyl adipate, occupational workers should be careful and use self-contained breathing apparatus, rubber boots, and heavy rubber gloves and avoid prolonged period of exposures. Workers should avoid contact of dimethyl adipate with skin, eyes and nose. |