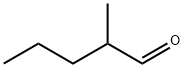
| Description | 2-Methylpentanal has an ethereal, fruity odor. This may be prepared from 2-methylpentanol by catalytic oxidation. |
| Chemical Properties | Colorless liquid. Soluble in water 0.42% by wt, flash p68F (20C) (OC). |
| Occurrence | Reported found in onion, leek, garlic, milk, roasted peanuts, wheat bread, cooked beef, cured pork, coffee, beer, tea, trassi, rice and mango. |
| Uses | Intermediates for dyes, resins, pharmaceuticals. |
| Uses | 2-Methylvaleraldehyde is used as an intermediate to manufacture organic compounds (pharmaceuticals, odorants and flavorings) |
| Preparation | From 2-methylpentanol by catalytic oxidation. |
| Definition | ChEBI: 2-Methylpentanal is a 2-methyl-branched fatty aldehyde. |
| Aroma threshold values | Detection: 1.6 to 3.2 ppb |
| Taste threshold values | Taste characteristics at 5.0 ppm: vegetative and green with a fruity, grape-like nuance. |
| Synthesis Reference(s) | Synthesis, p. 717, 1985 DOI: 10.1055/s-1985-31327 |
| General Description | A colorless liquid. Less dense than water. Flash point near 50°F. Used to make rubber and artificial flavorings. |
| Air & Water Reactions | Highly flammable. Slightly soluble in water. |
| Reactivity Profile | Methyl valeraldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. |
| Hazard | Flammable, dangerous fire risk. Strong irri-tant to skin and mucous membranes. |
| Health Hazard | May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
| Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Flammability and Explosibility | Highlyflammable |