| Hydroxyacetone Basic information |
| Product Name: | Hydroxyacetone |
| Synonyms: | Hydroxyacetone contains <=500 ppM sodiuM carbonate as stabilizer, technical grade, 90%;Hydroxyacetone, 95%, 95%;Acetol 〔Hydroxyacetone〕;1-Hydroxypropan-2-one, Acetol;3-hydroxy-4-oxopentanoic acid;Hydroxyacetone, Technical 100GR;Acetol Hydroxy-2-propanone;HYDROXYACETONE |
| CAS: | 116-09-6 |
| MF: | C3H6O2 |
| MW: | 74.08 |
| EINECS: | 204-124-8 |
| Product Categories: | ketone Flavor;Industrial/Fine Chemicals |
| Mol File: | 116-09-6.mol |
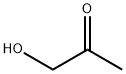 |
| Hydroxyacetone Chemical Properties |
| Melting point | -17 °C (lit.) |
| Boiling point | 145-146 °C (lit.) |
| density | 1.082 g/mL at 25 °C (lit.) |
| vapor pressure | 9.01hPa at 25℃ |
| FEMA | 4462 | HYDROXYACETONE |
| refractive index | n20/D 1.425(lit.) |
| Fp | 133 °F |
| storage temp. | 2-8°C |
| solubility | water: miscible |
| pka | 13.14±0.10(Predicted) |
| form | clear liquid |
| color | Colorless liquid |
| Specific Gravity | 1.090 (20/4℃) |
| Odor | at 10.00 % in dipropylene glycol. pungent sweet caramellic ethereal |
| Odor Type | caramellic |
| Water Solubility | Miscible with water, alcohol and ether. |
| Sensitive | Hygroscopic |
| Merck | 14,65 |
| JECFA Number | 1945 |
| BRN | 605368 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture – hygroscopic. |
| InChIKey | XLSMFKSTNGKWQX-UHFFFAOYSA-N |
| LogP | 0.3 at 25℃ |
| CAS DataBase Reference | 116-09-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanone, 1-hydroxy-(116-09-6) |
| EPA Substance Registry System | 1-Hydroxyacetone (116-09-6) |
| Safety Information |
| Risk Statements | 2017/10/16 |
| Safety Statements | 23-24/25-5 |
| RIDADR | UN 1224 3/PG 3 |
| WGK Germany | 1 |
| RTECS | UC2800000 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29144090 |
| Toxicity | mmo-sat 500 mg/plate ABCHA6 47,2461,83 |
| Provider | Language |
|---|---|
| Acetol | English |
| SigmaAldrich | English |
| ALFA | English |
| Hydroxyacetone Usage And Synthesis |
| Chemical Properties | colourless to yellow liquid |
| Uses | Reagent in organic synthesis; protecting group for the synthesis of peptides. |
| Uses | Hydroxyacetone is used as a reagent in organic chemical reactions. It also serves as a component for Mannich reaction and aldol reactions. It is also used in the syntheses of 2-oxo-propionaldehyde, imidazoles, polyols, acrolein, dyes and skin tanning agents. It yields (R)-1,2-propanediol upon reduction of hydroxyacetone in the presence of a microbial cell catalyst. |
| Uses | Hydroxyacetone is a chemical reagent used in various organic chemical reactions. It is a component of the Mannich reaction, amino acid caalyzes direct asymmetric aldol reactions. In the pharmaceutical setting, this compound is used in the synthesis of imidazoles acting as potent and orally active antihypertensive agents. |
| Definition | ChEBI: A propanone that is acetone in which one of the methyl hydrogens is replaced by a hydroxy group. |
| General Description | Hydroxyacetone (Acetol) is important for the manufacture of polyols, acrolein, dyes and skin tanning agents. It undergoes asymmetric reduction to yield (R)-1,2-propanediol in the presence of microbial cell catalyst. |
| Safety Profile | Moderately toxic by ingestion. Mutation data reported. An allergen. Implicated in aplastic anemia. A 10 gram dose may be fatal to an adult. skin contact, inhalation, or ingestion can cause asthma, sneezing, irritation of eyes and nose, hives, and eczema. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and fumes. |
| Hydroxyacetone Preparation Products And Raw materials |