| Description | LY-171883 (88107-10-2) is a selective and orally active leukotriene D4 (LTD4) antagonist (Ki = 0.63 μM).1 It is also an activator of peroxisome proliferator-activated receptors (PPARs).2,3 |
| Uses | LY 171883 is a selective leukotriene D4 antagonist. |
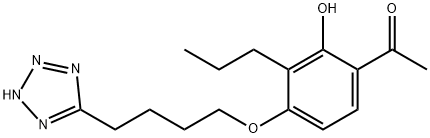
| Definition | ChEBI: A member of the class of acetophenones that is 1-phenylethanone substituted at position 2 by a hydroxy group, a propyl group at position 3 and a 4-(1H-tetrazol-5-yl)butoxy group at position 4. A leukotriene antagonist, it exhibits anti asthmatic activity. |
| Biological Activity | Selective, orally active leukotriene D 4 (LTD 4 ) antagonist; inhibits binding of [ 3 H]-LTD 4 to guinea pig lung membranes (K i = 0.63 μ M).? Acts as an agonist at peroxisome proliferator-activated receptors (PPARs). |
| in vitro | in gh(3) cells, ly-171883 was able to reversibly increase the amplitude of ca(2+)-activated k(+) current concentration-dependently with an ec(50) value of 15 μm. moreover, the treatment of ly-171883 to cytosolic face did not affect single channel conductance of large-conductance ca(2+)-activated k(+) channels in excised inside-out patches recorded from gh(3) cells, however, ly-171883 did increase the channel activity. in addition, the ly-171883-stimulated activity of bk(ca) channels was dependent on membrane potential [1]. |
| in vivo | the effect of ly-171883 on the respiratory and cardiovascular changes in endotoxemia was studied in unanesthetized sheep. in group one, ly-171883 at 4 mg/kg was i.v. injected. in group two, escherichia coli endotoxin (1 μg/kg) was infused, and in group three, ly-171883 at 4 mg/kg was administered before and after the same dose of endotoxin. results showed that infusion of ly-171883 in group one did not alter baseline ventilatory and cardiovascular measurements. in group two, a two-phase pulmonary response was found. an early pulmonary hypertension phase with a fall in cardiac index was observed in group three [2]. |
| References | 1) Fleisch et al. (1985), LY171883, 1-less than 2-hydroxy-3-propyl-4-less than 4-(1H-tetrazol-5-yl)butoxy greater than phenyl greater than ethanone, an orally active leukotriene D4 antagonist; J. Pharmacol. Exp. Ther., 233 148 2) Foxworthy and Eacho (1991) Effect of the peroxisome proliferator LY171883 on triglyceride accumulation in rats fed a fat-free diet; Biochem. Pharmacol. 42 1487 3) Kliewer et al. (1994) Differential expression and activation of a family of murine peroxisome proliferator-activated receptors; Proc. Natl. Acad. Sci .USA 91 7355 |