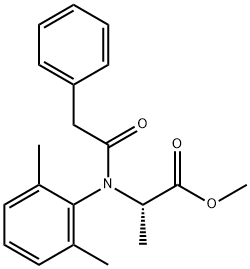
| Benalaxyl Basic information |
| Product Name: | Benalaxyl |
| Synonyms: | METHYL2-(N-PHENYLACETYL-N-(2,6-DIMETHYLPHENYL)AMINO)PROPANOATE;N-(2,6-dimethylphenyl)-N-(phenylacetyl)-DL-alanine;methyl-N-phenylacetyl-N-2,6-dimethyl phenyl-DL-alaninate;TF 3675;benalaxyl methyl N-(2,6-dimethylphenyl)-N-(phenylacetyl)-DL-alaninate;Benalaxyi;Benalaxyl 250mg [71626-11-4];(±)-N-(2,6-Dimethylphenyl)-N-(phenylacetyl)alanine methyl ester |
| CAS: | 71626-11-4 |
| MF: | C20H23NO3 |
| MW: | 325.4 |
| EINECS: | 275-728-7 |
| Product Categories: | A-BPesticides;Alpha sort;Amide structureAlphabetic;B;BA – BH;Fungicides;Pesticides&Metabolites;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals;FUNGICIDE |
| Mol File: | 71626-11-4.mol |
 |
| Benalaxyl Chemical Properties |
| Melting point | 78-80°C |
| Boiling point | 463.59°C (rough estimate) |
| density | 1.2700 |
| vapor pressure | 6.6 x 10-5 Pa (25 °C) |
| refractive index | 1.5614 (estimate) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| Water Solubility | 28.6 mg l-1 (20 °C) |
| pka | 1.52±0.50(Predicted) |
| form | neat |
| Merck | 13,1028 |
| BRN | 3001587 |
| NIST Chemistry Reference | Benalaxyl(71626-11-4) |
| EPA Substance Registry System | Benalaxyl (71626-11-4) |
| Safety Information |
| Hazard Codes | N |
| Risk Statements | 50/53 |
| Safety Statements | 60-61 |
| RIDADR | UN 3077 |
| WGK Germany | 2 |
| RTECS | AY5993200 |
| HS Code | 29242990 |
| Toxicity | LD50 in rats (mg/kg): 4200 orally; 1100 i.p.; LC50 (96 hr) in rainbow trout: 3.75 mg/l (Bergamaschi) |
| Benalaxyl Usage And Synthesis |
| Uses | Agricultural fungicide. |
| Uses | An acylamino acid fungicide. |
| Uses | Benalaxyl is used for the control of Oomycetes on a wide range of crops, fruit, flowers, ornamentals and turf. |
| Definition | ChEBI: Methyl N-(2,6-dimethylphenyl)-N-(phenylacetyl)alaninate is an alanine derivative that is the N-phenylacetyl derivative of methyl N-(2,6-dimethylphenyl)alaninate It is an alanine derivative, an aromatic amide, a carboxamide and a methyl ester. |
| Metabolic pathway | The fate of 14C-benalaxyl as affected by mancozeb is determined on the surface and in the tissues of grape leaves 7 days after the single foliar application of a mixture with mancozeb. The main oxidative scheme with rat liver microsomes is quite similar to that observed in plants. The only differences are represented by a further hydroxylation on the second ortho methyl group, and only traces of the meta hydroxylation take place on the xylene ring. |
| Degradation | Benalaxyl is stable in aqueous media over a wide pH range but it is hydrolysed in concentrated alkali. Its DT50 at pH 9 and 25 °C is 86 days (PM). It is reasonably stable to sunlight in aqueous solution. When irradiated in solution with UV light (254 nm) it is degraded with a half-life of 55 minutes (Pirisi et al., 1996). Products were compounds 2 and 3 (see Scheme 1) which are common also to metalaxyl and furalaxyl. These were detected in a non-labelled study. Under simulated sunlight its half-life was 167 days. |
| Benalaxyl Preparation Products And Raw materials |
| Raw materials | Nickel–>Cyclohexane–>2,6-Dimethylaniline–>Methyl propionate–>Methyl acetate–>Phenylacetyl chloride–>methyl N-(2,6-dimethylphenyl)-DL-alaninate |
| Preparation Products | Benalaxyl-M |