| Corrosivity | The chemical and formulations containing the chemical applied to intact rabbit skin produced severe erythema, oedema and scar formation. The exposed areas had effects that were not reversible. Data indicates corrosive effects in the respiratory tract. While no data are available for effects in the eyes, the chemical is deemed to capable of causing severe damage. There is sufficient evidence to warrant hazard classification. |
| Description | As an irritant and sensitizer, Kathon 930 caused contact dermatitis in employees of a textile finishing factory. |
| Chemical Properties | solid |
| Application | Suitable for applications in the paints and coatings, wood, and leather fields to resist corrosion and protect against mildews and algae. |
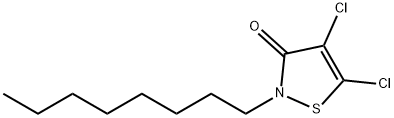
| Definition | ChEBI: A 1,2-thiazole that is 1,2-thiazol-3(2H)-one substituted by chloro groups at positions 4 and 5 and an octyl group at position 2. It is used as a fungicide. |
| Health Hazard | The chemical is referred to as 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) in this assessment. Where available, data for two commercial antifouling products C-9211 HQ (32.6 % of DCOIT in xylene) and Acticide? DCOIT (97.1% purity) are used for some health end-points. The information on health hazards is primarily obtained from the comprehensive reviews from the Norwegian Environment Agency as part of its ECHA CLH proposal (ECHA, 2018) and an EU (Norway) DCOIT evaluation for the use of DCOIT as a biocide in antifouling products (EU, 2014). Unless otherwise noted, references to individual studies below are taken from these reviews. |
| Contact allergens | Irritant and sensitizer, Kathon? 930 caused contact dermatitis in employees of a textile fnishing factory. |
| Toxicology | The chemical, DCOIT is moderately absorbed through skin and moderately absorbed via oral route. It is then extensively distributed to tissues (liver, kidney, stomach and intestine) and metabolised following oral administration. In a study conducted in rats, 81–93 % of orally administered 14C-DCOIT was excreted primarily in the faeces within a 2-day period. Plasma elimination half-life of 14C-DCOIT was 16.1–19.4 hours for males and 20.5–25.0 hours for females. The highest concentration of 14C-DCOIT was found in liver, stomach, intestine and kidney. More than 80 % of the administered dose was eliminated via faeces, 11–18 % was eliminated via urine and less than 2 % of the dose was eliminated through exhaled air. DCOIT metabolised to form six metabolites in the faeces and eight metabolites in the urine. Degradation of DCOIT involves cleavage of the ring and subsequent oxidation of 7–18 % of the administrated dose to N-(n-octyl) malonamic acid (NNOMA, major metabolite), N-(n-octyl)acetamide, N-(n-octyl)oxamic acid and N-(n-octyl)-β-acetyl propionamide. The chemical undergoes subsequent biotransformation involving hydroxylation, dealkylation and acetylation (EU, 2014; ECHA, 2018). |
| Synthesis | Add 200 g of ethyl acetate and 23.6 g (0.1 mol) of N,N’-dimethyl-3,3′-dithiodipropionamide into a 500ml four-neck flask equipped with a thermometer and a stirrer, stir and mix, and control The reaction temperature was 15° C., 1.8 g of thionyl chloride (15.1 mmol) was added to the four-neck flask, and the mixture was stirred for 10 min.21.3g (0.3mol) of chlorine gas was introduced into the reaction system, the mixture was stirred for 5h to react, and 27.1g of 4,5-Dichloro-2-octyl-isothiazolone was obtained by filtration and separation. The yield was 84.9%. |
| in vitro | In a bacterial gene mutation test, DCOIT was tested in Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537 up to a maximum concentration of 300 μg/plate with negative responses in all strains with and without metabolic activation (EU, 2014; ECHA, 2018). |
| in vivo | In two micronucleus assays, DCOIT was tested in CD-1 mice (5-9 mice/sex/dose) at doses up to 325 mg/kg bw/day by gavage. No genotoxicity was reported in both studies (EU, 2014; ECHA, 2018). |
| Precautions | 1. Suitable for use in all marine paints. Can effectively kill many bacteria and mildews like penicillium notatum, basidiomycetes, aspergillus niger, fusarium, and curvularia. Can be added at any production step for use; it is recommended to adopt the concentration of 5 ~ 15% (w/w).2. Operational instruction must be strictly followed. Avoid any contact with the skin. Wear protective clothes, goggles, and rubber gloves in operation. Once any contact with the skin happens, wash the skin immediately with plenty of water and soaps; when it splashes into eyes, wash immediately with plenty of water and buffer solutions. |