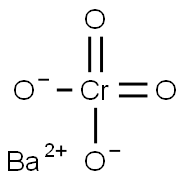
| Chemical Properties | Barium chromate is a heavy, yellow powder(s); rhomb, monoclinic; prepared from BaCl2 and Na2CrO4 solutions, followed by filtering resulting precipitate; used in safety matches, as a corrosion inhibitor.[MER06] [HAW93] |
| Physical properties | Yellow orthorhombic crystal; density 4.50 g/cm3; darkens on heating; insoluble in water and organic solvents; dissolves in mineral acid with decomposition. |
| Uses | Barium chromate has been found to be useful in many capacities. It is an oxidizing agent, making it useful as a burn rate modifier in pyrotechnic compositions. It is especially useful in delay compositions such as delay fuses. Barium chromate is used as a pigment, oxidizing agent, colorant in glass, and sulfate scavenger in chromium electroplating baths, ceramics, and porcelain. It acts as a corrosion inhibitor in jointing pastes and metal primers. Further, it is used in fuses, safety matches, ignition control devices and high-temperature batteries. In addition to this, it is used as a carrier for the chromium ions and useful as a burn rate modifier in pyrotechnic compositions. It is involved as a catalyst in the alkane dehydration reaction. |
| Application | Barium chromate is an oxidizing chemical compound composed of the elements barium and chromium. It is a pigment almost entirely in anticorrosion jointing pastes to prevent electro-chemical corrosion at junctions of dissimilar metals; some use in artists’ colors and in coloring glass, ceramics, porcelain. Also used in metal primers, pyrotechnic compositions. |
| Preparation | Interaction of barium chloride and sodium chromate. The precipitate is washed, filtered and dried.
BaCl2+Na2CrO4→BaCrO4↓+2NaCl |
| Hazard | Confirmed carcinogen. |
| Safety Profile | Confirmed human carcinogen. A poison. Mutation data reported. Reacts vigorously with reducing materials. See also BARIUM COMPOUNDS (soluble) and CHROMIUM COMPOUNDS. Used in pyrotechnics and as an explosive initiator. |