| Carbon Black Basic information |
| Production method Uses Identification test Content analysis Toxicity Use the limit Chemical properties Hazards & Safety Information |
| Product Name: | Carbon Black |
| Synonyms: | NORIT SA II;NORIT(R) SX-2;NORIT(R) SA II;NORIT(R) ROW 0.8;NORIT(R) RO 0.8;NORIT(R) GSX;NORIT(R) CA1;NORIT(R) A SUPRA |
| CAS: | 1333-86-4 |
| MF: | C |
| MW: | 12.01 |
| EINECS: | 215-609-9 |
| Product Categories: | Packed GC;Packings, Uncoated;UVCBs-organic;metal or element;carbon;7440-44-0;Activated Carbon;Inorganic Chemicals;Industrial/Fine Chemicals;1333-86-4 |
| Mol File: | 1333-86-4.mol |
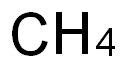 |
| Carbon Black Chemical Properties |
| Melting point | 3550 °C(lit.) |
| Boiling point | 500-600 °C(lit.) |
| density | ~1.7 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| Fp | >230 °F |
| solubility | H2O: soluble0.1mg/mL |
| form | rod |
| color | Clear colorless |
| Specific Gravity | bulk 0.10/g/cm3 |
| Water Solubility | Insoluble |
| Merck | 14,1808 |
| Exposure limits | ACGIH: TWA 3 mg/m3 OSHA: TWA 3.5 mg/m3 NIOSH: IDLH 1750 mg/m3; TWA 3.5 mg/m3; TWA 0.1 mg/m3 |
| Stability: | Stable. Combustible. |
| InChIKey | VNWKTOKETHGBQD-UHFFFAOYSA-N |
| LogP | 1.090 (est) |
| IARC | 2B (Vol. Sup 7, 65, 93) 2010 |
| EPA Substance Registry System | Carbon black (1333-86-4) |
| Safety Information |
| Hazard Codes | |
| WGK Germany | 3 |
| RTECS | FF5250100 |
| TSCA | Yes |
| Hazardous Substances Data | 1333-86-4(Hazardous Substances Data) |
| Toxicity | LD50 oral in rat: > 15400mg/kg |
| IDLA | 1,750 mg/m3 |
| Provider | Language |
|---|---|
| Carbon Black | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Carbon Black Usage And Synthesis |
| Production method | Natural gas tank method of making carbon black: take natural gas as raw material and use iron pipe to send it into the combustion chamber. The form of the combustion chamber can be either long and short and is made of iron plate. It contains a number of olefin burner inside it. Natural gas is sprayed with appropriate force from the burner nozzle and burned in the case of insufficient air, that is, to generate a bright and black smoke flame. The flame then goes directly into the channel iron with the distance between the burner and the slot surface being 65~80 mm. At this time, the temperature of olefin burning is reduced from about 1000 to 1400 ° C to about 500 ° C, and the carbon black is accumulated. The groove can move back and forth horizontally, with a moving speed of 3 to 4 mm/s. In order to maintain normal production, the required amount of air is about 2.5 to 3 times the theoretical calculation. The resulting carbon black was scraped into a funnel with a fixed doctor blade and sent to a central packing chamber for disposal. Then the carbon black is softened, filtered to remove the hard particles and scale and further sent into the mill grinding to enable more uniform thickness. However, the body is still very light and loose, thus should be shaken to a become a bit solid. Then add a small amount of water to the carbon black to make it into paste-like shape and have a small needle rotated inside it to forming micro-pellets, followed by drying to obtain the finished product. In the case of using pigment for carbon black, in order to facilitate the dispersion, the granulation is unnecessary. The process is as follows: Raw gas, air → combustion cracking → collection → granulation → packaging → finished product. Carbon black is one of the oldest industrial products. In ancient times, china has already applied incomplete combustion of vegetable oil for making pigment carbon black. In 1872, the United States first used natural gas as raw material to produce carbon black using tank method and mainly used it as a coloring agent. It was not until 1912 when Mott found the reinforcement effect carbon black on the rubber before the carbon black industry had gotten rapid development. Then it had successively developed of a variety of process methods. At present, oil furnace method is the most efficient and most economical method with the oil furnace black production amount accounting for 70-90% of the total carbon black production. There are mainly furnace, slot method, thermal cracking, three methods. It is obtained by the carbonization of the plant material such as peat. It can also be derived from the carbonization of cocoa shell and beef bone or from the combustion of vegetable oil. |
| Uses | 1. It is edible black pigment. It can be used for pastry with the usage amount of 0.001% to 0.1%. 2. It can be used for food coloring agent. China provides that it can be used for rice, flour products, candy, biscuits and pastries with the maximum usage amount of 5.0g/kg. 3. Rubber industry uses it as a reinforcing filler. 2. Paint Inks applies it as coloring pigments in paint inks. 3. Used for the manufacturing of black paper such as packaging materials for photographic materials and the black paper made of high-conductivity black carbon in the radio equipment. 4. Carbon paper and typewriter; it is used when it is required for darker colors and can remain on the carrier. 5. Plastic coloring, ink, phonograph records, shoe polish, paint cloth, leather coatings, colored cement, electrodes, electronic brushes, batteries and so on. 4. As electric conductive agent of lithium ion battery; 5. Mainly used for rubber, paint, ink and other industries; 6. Used for the reinforcement of car tread and sidewall, hose, groove, industrial rubber products as well as conveyor belt. 7. Used for tire tread, surface tire repair, automotive rubber parts, conveyor belts, conveyor pads, etc., The vulcanized glue of this carbon black shows excellent tensile strength and abrasion resistance 8. It is mainly used for the reinforcement of tire belt, sidewall, solid tires, outer layer of roller, hose surface, industrial rubber products and car tire tread. 9. It is used for the reinforcement of the tire tread of car and truck, surface of conveyor belt and industrial rubber products. 10. For rubber reinforcement, coloring agent, metallurgy, rocket propellant 11. For rubber products to fill and reinforcement. 12. For rubber products, carcass, valves and other filling . 13. For paints and inks, plastics and other industries. 14. Mainly used for raw materials of battery as well as for conductive and anti-static rubber products. 15. In the rubber industry, it is used as the reinforcing agent and filter for the manufacturing of natural rubber and butyl rubber, being able to endow the vulcanized rubber with excellent tensile strength, elongation and tear resistance and so on. It should be mostly used for natural rubber-based large-scale engineering tires and a variety of off-road tires as well as being used for carcass and sidewall. In addition, it can also be used for high-strength conveyor belt, cold rubber products and drilling device. In light industry, it can be used as the filter of the paint, ink, enamel and plastic products. |
| Identification test | Solubility: being insoluble in water and organic solvents (OT-42) Heated to red, burning without flames. |
| Content analysis | The sample was pre-dried at 120 ° C for 4 h and then measured by an instrument such as a C.H. O analyzer or subject to combustion/gravimetric analysis. |
| Toxicity | ADI has not yet been specified. It is listed as substance allowed to be in temporary contact with food, (FAO/WHO, 2000). It can not be digested and absorbed, so oral administration should be non-toxic, but given the incorporation of 3, 4-benzopyrene during the carbonization, it is basically not used now. |
| Use the limit | GB 2760-1996: Confectionery, biscuits, pastries, rice and flour products, 5.0 g/kg. EEC provides for being used for concentrated fruit juice, jam, jelly, fruit wine. |
| Chemical properties | It appears as black powdery particles with a particle size of 0 to 500 μm. The relative density is 1.8 to 2.1. It is insoluble in water and organic solvents. |
| Hazards & Safety Information | Category Toxic substances Toxicity classification Low toxicity Acute Toxicity Oral-Rat LD50:> 15400 mg/kg Explosives and hazardous characteristics being explosive upon dust and air mixture Flammability and Hazardous characteristics It is combustible in case of heat and strong oxidant Storage and transportation characteristics Treasury: low temperature, ventilated and dry Fire extinguishing agent water, carbon dioxide, dry powder, foam Occupational Standard TWA 3.5 mg/m3; STEL 7 mg/m3 |
| Description | Carbon black is a finely divided form of carbon. It may ignite explosively if suspended in air in the presence of an ignition source or slowly undergo spontaneous combustion upon contact with water. In addition, it is toxic by inhalation, with a TLV of 3.5 mg/m3 in air. Primary uses are in the manufacture of tires, belt covers, plastics, carbon paper, colorant for printing inks, and as a solar-energy absorber. |
| Chemical Properties | Carbon black (essentially elemental carbon), is a black or brown liquid or solid (powder). Odorless solid. Carbon black oil is flammable and has a petroleum odor. |
| Chemical Properties | finely divided black dust or powder |
| Physical properties | Carbon black [1333-86-4] is virtually pure elemental carbon (diamond and graphite are other forms of nearly pure carbon) in the form of near-spherical colloidal particles that are produced by incomplete combustion or thermal decomposition of gaseous or liquid hydrocarbons. Its physical appearance is that of a black, finely divided pellet or powder, the latter sometimes small enough to be invisible to the naked eye. Its use in tires, rubber and plastic products, printing inks and coatings is related to the properties of specific surface area, particle size and structure, conductivity and color. It is in the top 50 industrial chemicals manufactured worldwide, based on annual tonnage. Current worldwide production is about 15 billion pounds per year (6.81 million metric tons). Approximately 90% of carbon black is used in rubber applications, 9% as a pigment, and the remaining 1% as an essential ingredient in hundreds of diverse applications. Modern carbon black products are direct descendants of early “lampblack”, first produced in China over 3500 years ago. These early lampblacks were not very pure and differed greatly in their chemical composition from current carbon blacks. Since the mid-1970s most carbon black has been produced by the oil furnace process, which is most often referred to as furnace black. Unlike diamond and graphite, which are crystalline carbons, carbon black is an amorphous carbon composed of fused particles called aggregates. Properties, such as surface area, structure, aggregate diameter and mass differentiate the various carbon black grades. |
| Uses | In the rubber, plastic, printing, and paint industries as a reinforcing agent and a pigment |
| Uses | Tire treads, belt covers, and other abrasion- resistant rubber products; plastics as a reinforc- ing agent, opacifier, electrical conductor, UV- light absorber; colorant for printing inks;carbon paper; typewriter ribbons; paint pigment; nucleat- ing agent in weather modification; expanders in bat- tery plates; solar-energy absorber (see note). |
| Uses | Carbon black Super-P (TIMCAL) was used as conductive agent. Super P furnace black the best conductive additive. Carbon black (conducting material, super P black) was added with binder in the composite electrode to compensate the low electrical conductivity of PPy and PPyDVB in miniemulsion polymerization. The hybrid Super P-SACNT conductive network manifests itself as a promising strategy to improve the battery performances with a minimum amount of conductive fillers. |
| Definition | carbon black: A fine carbon powdermade by burning hydrocarbons in insufficientair. It is used as a pigmentand afiller (e.g. for rubber). |
| Definition | A finely divided form of carbon produced by the incomplete combustion of such hydrocarbon fuels as natural gas or petroleum oil. It is used as a black pigment in inks and as a filler for rubber in tire manufacture. |
| Definition | A finely divided form of carbon, practically all of which is made by burning vaporized heavy-oil frac- tions in a furnace with 50% of the air required for complete combustion (partial oxidation). This type is also called furnace black. Carbon black can also be made from methane or natural gas by crack- ing (thermal black) or direct combustion (channel black), but these methods are virtually obsolete. All types are characterized by extremely fine particle size, which accounts for their reinforcing and pig- menting effectiveness. |
| General Description | Multi walled carbon nanotubes (MWNTs, CNTs) were prepared by chemical vapor deposition (CVD). In chemical vapor deposition (CVD), a volatile precursor undergoes thermal decomposition at elevated temperatures to form a solid deposit on a substrate. 1 Carboxylic acid groups can be attached to the defect sides and ends of the nanotube by treatment with oxidizing agents. Carboxylic acid groups can be easily derivatized into different functional groups. |
| Hazard | Possible carcinogen. Bronchitis. |
| Health Hazard | There are no well demonstrated health hazards to humans from acute exposure to carbon black. Commercial carbon black is a spherical colloidal form of nearly pure carbon particles and aggregates with trace amounts of organic impurities adsorbed on the surface. Potential health effects usually are attributed to these impurities rather than to the carbon itself. Soots, by contrast, contain mixtures of particulate carbon, resins, tars, and so on, in a nonadsorbed state. |
| Biochem/physiol Actions | The use of dextran-coated charcoal makes the immunoassay of insulin in biological fluids simpler and more rapid. In theory, the charcoal coated with dextran will adsorb the free hormone and leave hormones that are bound to a carrier (or antibody). Dextran coated charcoal is used to strip hormones from serum instead of charcoal alone, because there is less loss of protein using dextran coated charcoal. |
| Safety Profile | Mildly toxic by ingestion, inhalation, and skin contact. Questionable carcinogen. Mutation data reported. See also CARBON. A nuisance dust in high concentrations. We it is true that the tiny particulates of carbon black contain some molecules of carcinogenic materials, the carcinogens are apparently held tightly and are not eluted by hot or cold water, gastric juices, or blood plasma. |
| Potential Exposure | Used as reinforcing agent and filler for rubber; colorants for ink, paint, and plastics. Workers in carbon black production or in its use in rubber compounding, ink and paint manufacture, plastics compounding, drycell battery manufacture. |
| Shipping | Carbon black oil: UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required. |
| Properties and Applications | TEST ITEMSSPECIFICATIONAPPEARANCEBLACK POWDER / BEADSHADEBLUISHDBP ABSORPTION NUMBER99 cc/100gBET SURFACE AREA80 m 2 /gpH VALUE10.0AVERAGE ORIGINAL PARTICAL SIZE34 nmDENSITYg/lIMPURITYNO FOUNDVOLATITE2.0 %TINTING STRENGTH vs IRB#3100 % min |
| Incompatibilities | Carbon blacks containing over 8% volatiles may pose an explosion hazard. Dust can form an explosive mixture in air. A reducing agent; keep away from strong oxidizers, such as chlorates, bromates, nitrates. |
| Waste Disposal | Dump into a landfill or incinerate as a slurry. |
| Carbon Black Preparation Products And Raw materials |