| Flupirtine maleate Basic information |
| Description Painkiller References |
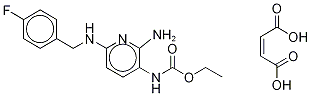
| Product Name: | Flupirtine maleate |
| Synonyms: | 2-AMINO-6-[[(4-FLUOROPHENYL)METHYL]AMINO]-3-PYRIDINYL]-CARBAMIC ACID, ETHYL ESTER MALEATE;Flupirtinemaleatelupirtine;Ethyl-2-amino-6-[(4-fluorbenzyl)amino]pyridin-3-carbamat, Verbindung mit Maleinsure (1:1);Keiton;Ketadolon;Flupirtine Maleate Salt;2-AMino-6-[(p-fluorobenzyl)aMino]-3-pyridinecarbaMic Acid Ethyl Ester Maleate;N-[2-AMino-6-[[(4-fluorophenyl)Methyl]aMino]-3-pyridinyl]carbaMic Acid Ethyl Ester (2Z)-2-Butenedioate |
| CAS: | 75507-68-5 |
| MF: | C19H21FN4O6 |
| MW: | 420.3916432 |
| EINECS: | 278-225-0 |
| Product Categories: | API;Inhibitors;Other APIs;Other Potassium channel modulators;APIS;Amines;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals |
| Mol File: | 75507-68-5.mol |
 |
| Flupirtine maleate Chemical Properties |
| Melting point | 186-188°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO: soluble >20mg/mL |
| form | solid |
| color | white |
| λmax | 343nm(CH3CN)(lit.) |
| Merck | 14,4190 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| InChIKey | DPYIXBFZUMCMJM-BTJKTKAUSA-N |
| Safety Information |
| Safety Statements | 22-24/25-35-20 |
| WGK Germany | 3 |
| RTECS | EY8555800 |
| HS Code | 29333990 |
| Flupirtine maleate Usage And Synthesis |
| Description | Flupirtine is a selective neuronal potassium channel opener (SNEPCO) that also has N-methyl-D-aspartate (NMDA) receptor antagonist property which has been shown to be effective in the management of body pain. Flupirtine is used as an analgesic for acute pain, in moderate-to-severe cases. Its muscle relaxant properties make it popular for back pain and other orthopedic uses, but it is also used for migraines, in oncology, postoperative care, and gynecology. It is also effective in other painful conditions in which the primary requirement for analgesia is without sedation or anti-inflammatory effects. It first became available in Europe in 1984 under the brand name Katadolon. Flupirtine is a derivative of triaminopyridine with a chemical formula of ethyl-N-{2-amino-6-(4-fluorophenylmethylamino) pyridine-3 yl} cabamate. It is available as the maleate salt because flupirtine is itself poorly water soluble. Flupirtine is a base and is weakly lipophilic. |
| Painkiller | At present, the pain medications, used in the clinical in China, are divided into two categories: a class is to morphine on behalf of narcotic analgesics, and the other is aspirin antipyretic analgesics as representative. While the former high pain intensity, but addiction is its Achilles heel, which greatly limits its application; but the latter, although with no addictive analgesic strength, but it is weak, and has serious gastrointestinal effects. Flupirtine Maleate, developed by Pliva,is subsidiary company German AWD, a new non-opioid analgesics central, listed in Germany in 1986 for the treatment of postoperative pain, dental pain, painful wounds and burns. Then in 1990, it was applied in the treatment of degenerative joint disease, neuropathic and cancer pain, migraine, headache and dysmenorrhea indications. After that, the flupirtine maleate list in Brazil and Portugal. China approved the company’s German AWD flupirtine maleate capsules imports in September in 2006, trade name: Kodak DoLong (Katadolon (R)). Flupirtine maleate is a selective neuronal potassium channel opener, oral easily absorbed, which has three efficacy about analgesic, muscle relaxant and neuroprotective. It showed analgesic effects to pain caused by a variety of reasons. Flupirtine maleate has no affinity to Ah receptor, which do not inhibit prostaglandin synthesis. It is not antagonistic naloxone. Pain intensity (ED50) was weaker than methadone, buprenorphine and morphine, about 50% of morphine. Comparable to that of pentazocine, Flupirtine maleate is better than pethidine, codeine, phenacetin and paracetamol. Such as in the hot plate test, the effect of the strength of flupirtine (ED50 of 32mg/Kg, oral administration) is about half of morphine, codeine is 2 times, 10 times stronger than paracetamol and phenacetin. In the wake of dog dental pulp stimulation experiment, the analgesic intensity (ED50 of 3.5mg/ Kg, oral administration) is same with pentazocine. Oral absorption of this product is about 90%, and rectal administration of this product is about 70%. After oral administration of 20~30min. It showed the pharmacological effects, and the duration of action is 3~5h. Plasma half-life is 8~11h. The drug is in the body tissues, mainly in the liver metabolism, and about 70% of the drug is eliminated by the kidneys. Flupirtine maleate is inhibited to pain caused by various diseases. the analgesic effect is between methadone and paracetamol. Centrally acting, but it did not inhibit the respiratory cardiovascular systems, and has no pharmacological activity impact for cough center, nor constipation and urinary retention. Long-term use does not produce tolerance and dependence. It has no painkillers side effects like other medicines on the market, such as addiction, gastrointestinal symptoms. Long term use after a few years the efficacy is not reduced, but can reduce the amount of taking. In short, flupirtine maleate has good clinical application prospect. |
| References | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3557425/ https://en.wikipedia.org/wiki/Flupirtine |
| Description | Flupirtine maleate is a centrally-acting, non-addicting analgesic agent somewhat more potent than aspirin, paracetamol and pentazocine. It is reported to be useful in the treatment of postoperative and dental pain. |
| Description | Flupirtine is an activator of voltage-gated potassium channel 7 (Kv7/KCNQ). It induces relaxation of preconstricted pulmonary arteries isolated from wild-type and serotonin transporter-overexpressing (SERT+) mice. Flupirtine (30 mg/kg per day) decreases mean right ventricular pressure and right ventricular hypertrophy in hypoxia-induced and SERT+ mouse models of pulmonary arterial hypertension. It increases the paw withdrawal threshold in a rat model of streptozotocin-induced diabetic neuropathy when administered at a dose of 10 mg/kg and increases paw withdrawal latency in a rat model of carrageenan-induced paw inflammation when used in combination with morphine. Flupirtine also indirectly antagonizes NMDA receptors via its effects on potassium channels. |
| Chemical Properties | Off-White Solid |
| Originator | Chemiewerk Homburg (Degussa) (W. Germany) |
| Uses | Analgesic. Substituted pyridine with central analgesic properties. A potassium channel opener. |
| Uses | Analgesic. Substituted pyridine with central analgesic properties. |
| Brand name | KATADOLAN |
| Biological Activity | Non-opioid analgesic with muscle relaxant properties. Activates K + channels and indirectly antagonizes NMDA receptors. Exhibits neuroprotective actions in a model of cerebral ischemia in mice and reduces apoptosis and necrosis induced by noxious stimuli. |
| References | 1) Azad?et al. (2004), The potassium channel modulator flupirtine shifts the frequency-response function of hippocampal synapses to favour LTD in mice; Neuro. Sci. Lett.,?370?186 2) Klinger?et al.?(2012),?Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine; Br. J. Pharmacol.,?166?1631 3) Osbourne?et al. (1998),?Flupirtine, a nonopioid centrally acting analgesic, acts as an NMDA antagonist; Gen. Pharmacol.,?30?255 |
| Flupirtine maleate Preparation Products And Raw materials |
| Preparation Products | Flupirtine |