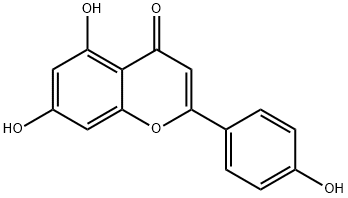
| Description | Apigenin is one of the most widespread flavonoids in plants and formally belongs to the flavone sub-class. Of all the flavonoids, apigenin is one of the most widely distributed in the plant kingdom, and one of the most studied phenolics. Apigenin is present principally as glycosylated in significant amount in vegetables (parsley, celery, onions) fruits (oranges), herbs (chamomile, thyme, oregano, basil), and plant-based beverages (tea, beer, and wine). Plants belonging to the Asteraceae, such as those belonging to Artemisia, Achillea, Matricaria, and Tanacetum genera, are the main sources of this compound. |
| Chemical Properties | Pale Yellow Crystalline Solid |
| Uses | Apigenin has been shown to possess antibacterial, antiviral, antifungal, and antiparasitic activities. Although it can’t stop all types of bacteria on its own, it can be combined with other antibiotics to increase their effects. |
| Uses | Apigenin is a promising reagent for cancer therapy. Apigenin appears to have the potential to be developed either as a dietary supplement or as an adjuvant chemotherapeutic agent for cancer therapy. |
| Uses | Apigenin is an active antioxidant, anti-inflammatory, anti-amyloidogenic, neuroprotective and cognitive enhancing substance with interesting potential in the treatment/prevention of Alzheimer’s disease. |
| Definition | ChEBI: Apigenin is a trihydroxyflavone that is flavone substituted by hydroxy groups at positions 4′, 5 and 7. It induces autophagy in leukaemia cells. It has a role as a metabolite and an antineoplastic agent. It is a conjugate acid of an apigenin-7-olate. |
| Preparation | 4-hydroxybenzaldehyde (1.22 g, 9.97 mmol, 1.0 equiv) was added to asolution of 50% KOH (aq.) (6.72 g, 59.82 mmol, 6.0 equiv) and ethanol (3 mL) andstirred for 10 min. Then compound 9 (2.02g, 9.97 mmol, 1.0 equiv) was added to the reaction mixture and heated to 60 °C and stirred for 4 h. After cooled toroom temperature, the mixture was poured into ice water and acidified withconcentrated hydrochloric acid to pH = 3. Then the suspension was filtrated, washed and the residue was dried toafford Apigenin(2.43 g, 90%) as a red solid. 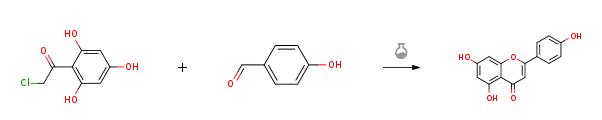 |
| Anticancer Research | It induces apoptosis by targeting leptin/leptin receptor pathway and by targeting caspase-dependent extrinsic pathway aswell as STAT3 signaling pathway in lung adenocarcinoma and BT-474 breast cancercells, respectively.
It shows antitumor activity against breastcancer MCF-7 cells and colon cancer HCT 116 cells and is a mediator of cancerchemoprevention and an inducer of autophagy. It can be used to treat colon canceras it induces apoptosis in colon cancer cells. It also increase melanogenesis in B16cells by activating the p38 MAPK pathway. |
| Purification Methods | The current method for the purification of apigenin is crude-solvent extraction method by using solvent in small quantity and also time saving. Apigenin that was isolated from Symphyotrichum novae-angliae was obtained in large quantity and directly from extract. |
| References | [1] Sanjeev Shukla, Sanjay Gupta (2010) Apigenin: a promising molecule for cancer prevention, 27, 962-978.
[2] https://en.wikipedia.org/wiki/Apigenin
[3] http://bodynutrition.org/apigenin/ |