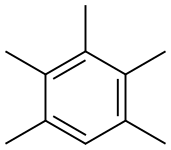
| Chemical Properties | Pentamethylbenzene is a white to light yellow crystalline powder with a sweet odor. The compound is classified as an aromatic hydrocarbon. It is a relatively easily oxidized benzene derivative, with E1/2 of 1.95 V vs NHE. |
| Uses | Pentamethylbenzene was used to prepare a mixture of nitropentamethylbenzene and 2,3,4,5-tetramethylbenzyl nitrate. It was also used to prepare propene. |
| Definition | ChEBI: Pentamethylbenzene is a methylbenzene that is benzene in which five of the hydrogens are replaced by methyl groups. |
| Synthesis Reference(s) | Synthesis, p. 979, 1985 DOI: 10.1055/s-1985-31413 |
| Synthesis | Pentamethylbenzene is obtained as a minor product in the Friedel–Crafts methylation of xylene to durene (1,2,4,5-tetramethylbenzene). Like durene, pentamethylbenzene is rather electron-rich and undergoes electrophilic substitution readily. Indeed, it is used as a scavenger for carbocations.
Pentamethylbenzene has been observed as an intermediate in the formation of hexamethylbenzene from phenol and alkylation of durene or pentamethylbenzene has been reported as a suitable starting material for the synthesis of hexamethylbenzene. |
| Purification Methods | Successively crystallise it from absolute EtOH, aqueous EtOH, MeOH, toluene *C6H6, and dry it under vacuum. [Rader & Smith J Am Chem Soc 84 1443 1962.] It has also been sublimed. The 1,3,5-trinitrobenzene complex (1:1) has m 121o (EtOH). [Beilstein 5 H 443, 5 III 1010, 5 IV 1109.] |
| Precautions | Incompatible with oxidizing agents. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. |