| 3-Nitrophthalonitrile Basic information |
| Product Name: | 3-Nitrophthalonitrile |
| Synonyms: | 2,3-DICYANONITROBENZENE;1,2-DICYANO-3-NITROBENZENE;3-NITRO-PHTHALODINITRILE;3-NITROPHTHALONITRILE;3-nitro-1,2-benzenedicarbonitrile;3-nitro-2-benzenedicarbonitrile;3-NITROPHTHALONITRILE 98+%;2,3-Dicyano-1-nitrobenzene |
| CAS: | 51762-67-5 |
| MF: | C8H3N3O2 |
| MW: | 173.13 |
| EINECS: | 450-650-8 |
| Product Categories: | Aromatic Nitriles;Benzene derivates;Functional Materials;Phthalonitriles & Naphthalonitriles;Phthalonitriles (Building Blocks for Phthalocyanines);N;Stains and Dyes;Stains&Dyes, A to |
| Mol File: | 51762-67-5.mol |
 |
| 3-Nitrophthalonitrile Chemical Properties |
| Melting point | 162-165 °C (lit.) |
| Boiling point | 386.1±37.0 °C(Predicted) |
| density | 1.41±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 20-25℃ |
| storage temp. | room temp |
| solubility | Methanol[soluble in] |
| form | Powder |
| color | White to Light yellow to Green |
| Water Solubility | Soluble in methanol. Insoluble in water. |
| BRN | 2263686 |
| LogP | 0.3 at 25℃ |
| Surface tension | 70.3mN/m at 212.5mg/L and 25℃ |
| CAS DataBase Reference | 51762-67-5(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2-Benzenedicarbonitrile, 3-nitro- (51762-67-5) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38-20/21/22 |
| Safety Statements | 26-36-45-37/39 |
| WGK Germany | 3 |
| RTECS | CZ1953200 |
| HS Code | 29269090 |
| Provider | Language |
|---|---|
| 3-Nitrophthalonitrile | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| 3-Nitrophthalonitrile Usage And Synthesis |
| Chemical Properties | light yellow powder |
| Uses | 3-Nitrophthalonitrile can be used in solar cells. Dyes and metabolites. |
| Application | 3-Nitrophthalonitrile belongs to nitrile derivatives and can be used as pesticide and pharmaceutical intermediates. It is an important intermediate for the synthesis of nitrophthalocyanine, nitrometallophthalocyanine or other phthalocyanine derivatives. |
| Preparation | The synthesis of 3-nitrophthalonitrile started with the nitration in position 3 of phthalimide followed by the formation of 3-nitrophthalamide; then dehydration by the thionyl chloride in N, N-dimethylformamide leads to 3-nitrophthalonitrile.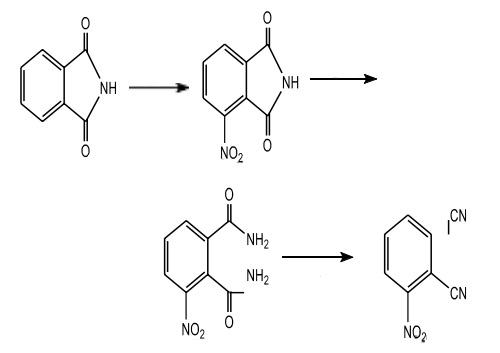 Synthesis of 3-Nitrophthalonitrile |
| Synthesis Reference(s) | Journal of Heterocyclic Chemistry, 32, p. 495, 1995 DOI: 10.1002/jhet.5570320219 |
| 3-Nitrophthalonitrile Preparation Products And Raw materials |